Top 10 Key Differences Between AS/NZS 4815 and AS 5369
Introduction
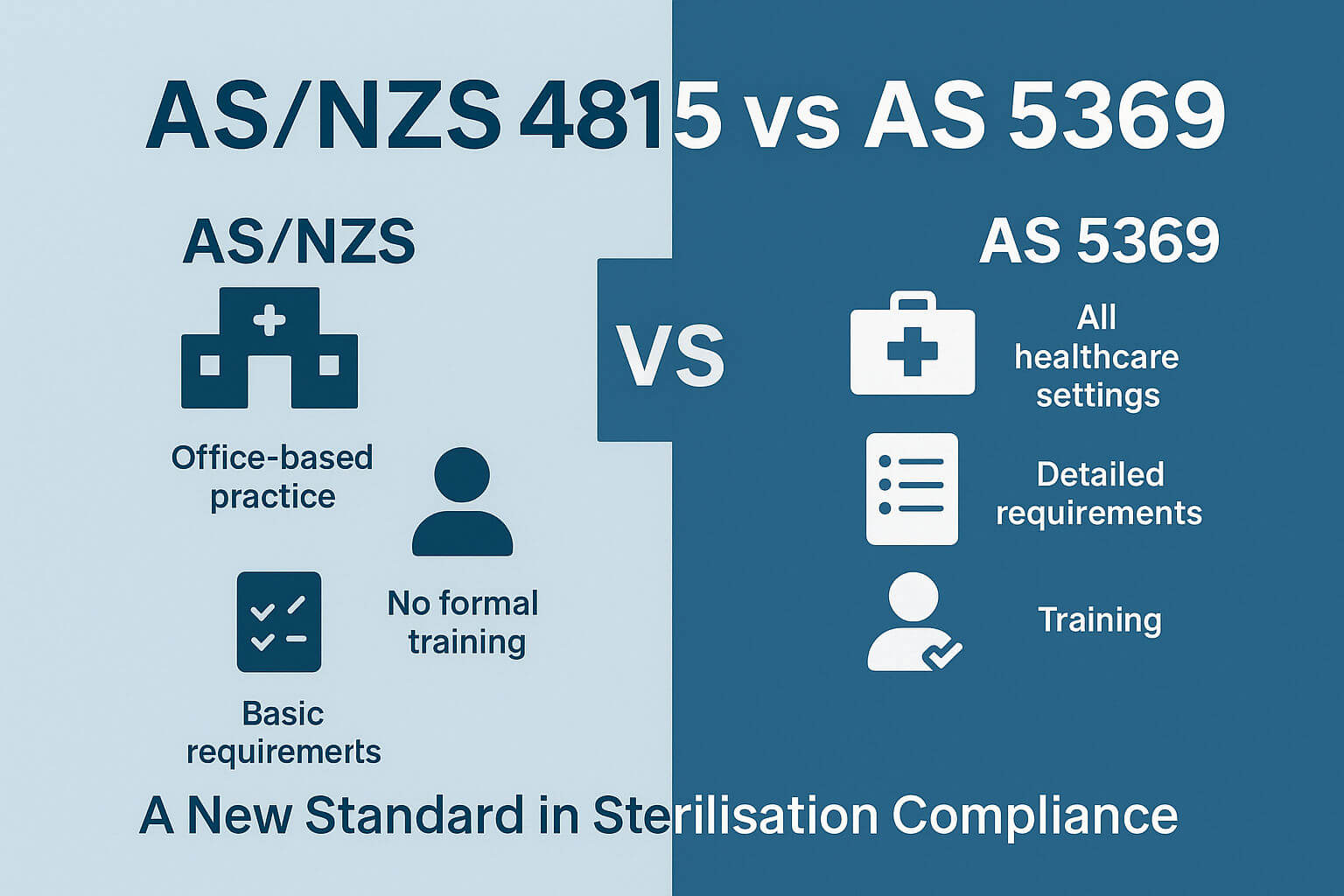
If your clinic or healthcare facility is still using AS/NZS 4815:2006, you're working under an outdated framework. The newly introduced AS 5369:2023 is a modernised, risk-based standard designed to enhance infection control and sterilisation practices in Australia. Below, we compare the top 10 differences between AS/NZS 4815 and AS 5369, with a focus on documentation compliance, staff training, and reprocessing protocols.
Read on to discover the Top 10 differences between the standards.




.png)









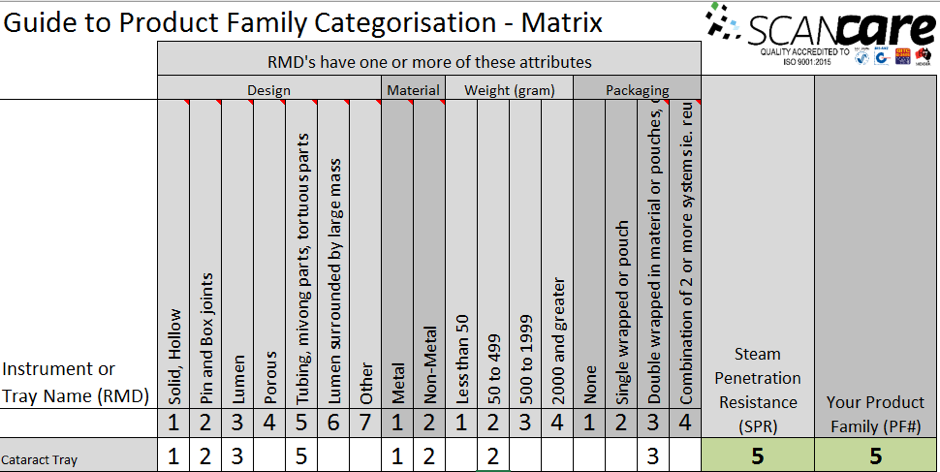



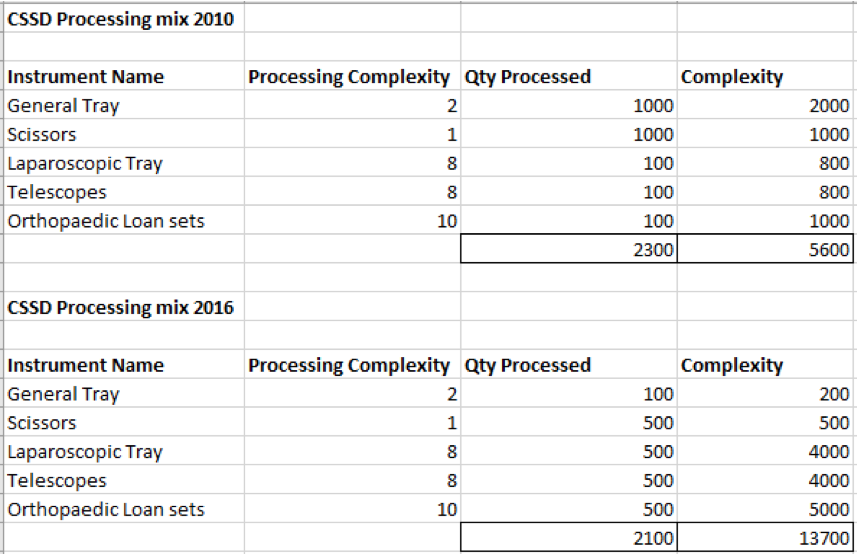

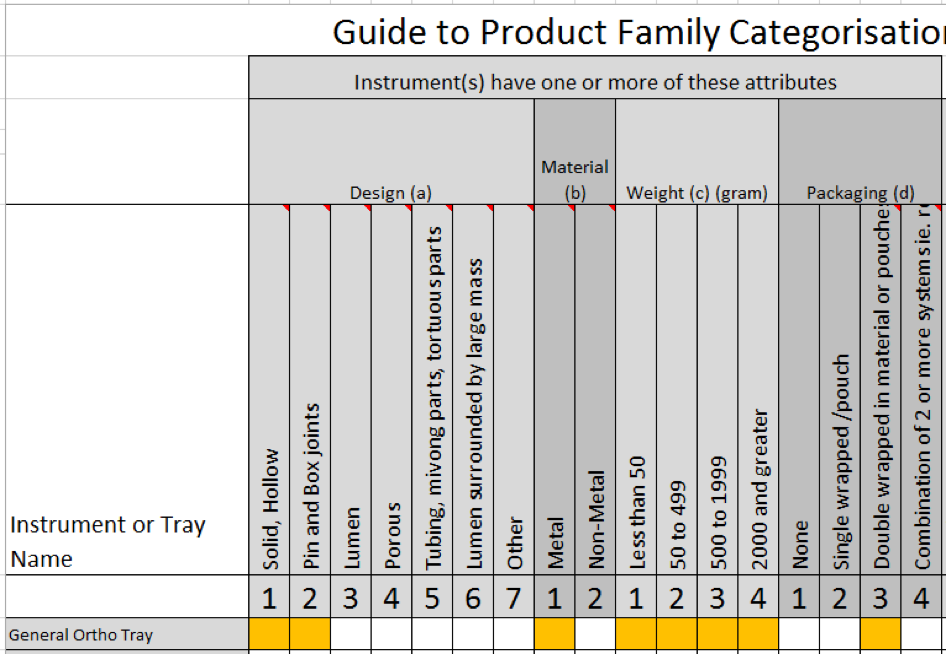



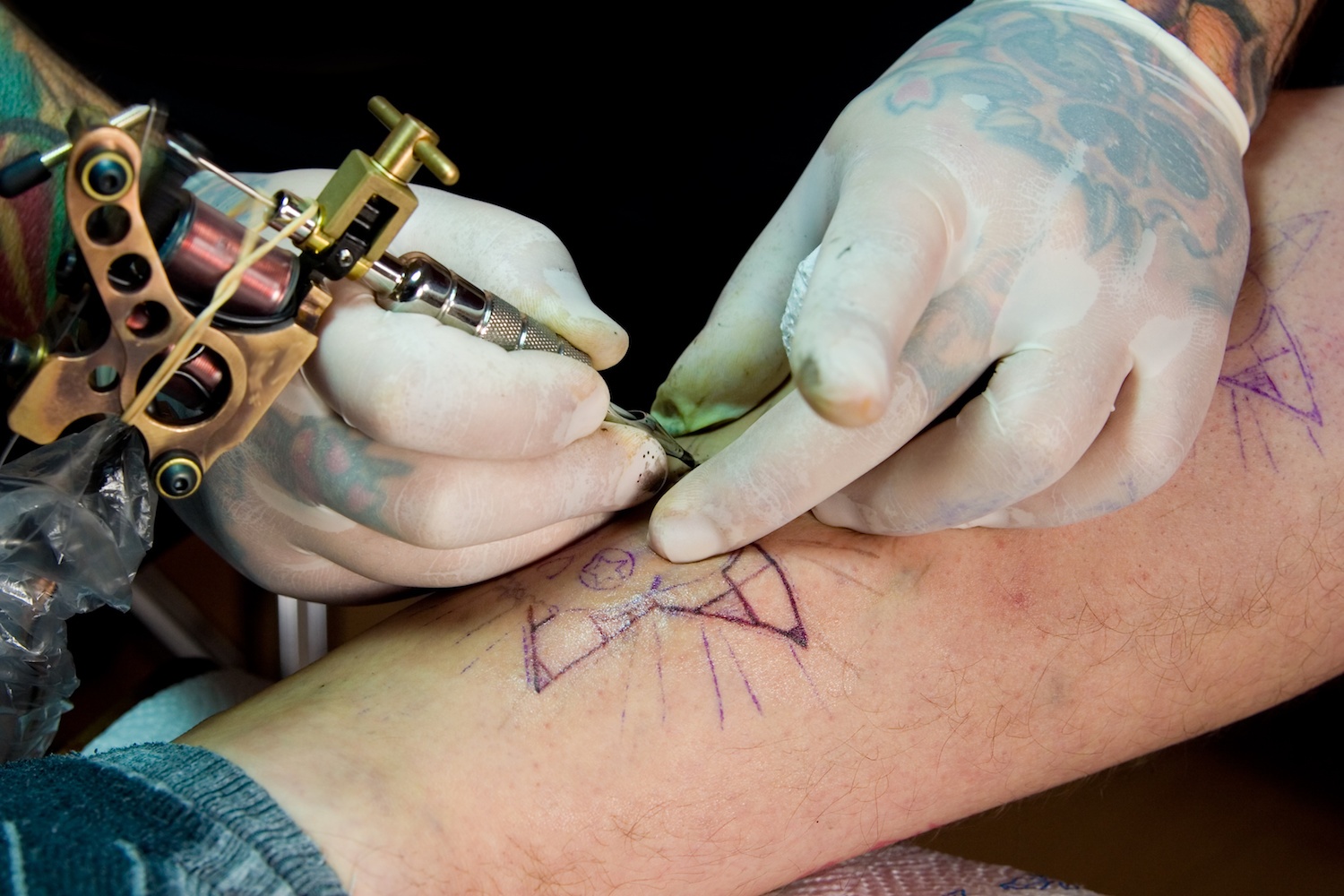

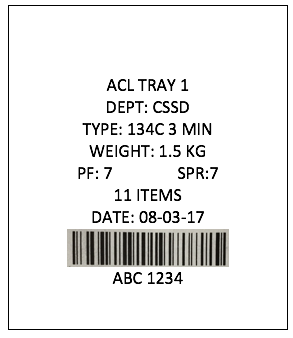
 Complying with Australian standards AS4187 or AS4815 can be a difficult task for any health care facility, but for office based health care facilities, physical space limitations can make it even more difficult. Finding space to wash, wrap and sterilize surgical instruments can be tricky for the smaller facilities and bench space is typically limited so finding space to put things can be a challenge.
Complying with Australian standards AS4187 or AS4815 can be a difficult task for any health care facility, but for office based health care facilities, physical space limitations can make it even more difficult. Finding space to wash, wrap and sterilize surgical instruments can be tricky for the smaller facilities and bench space is typically limited so finding space to put things can be a challenge.

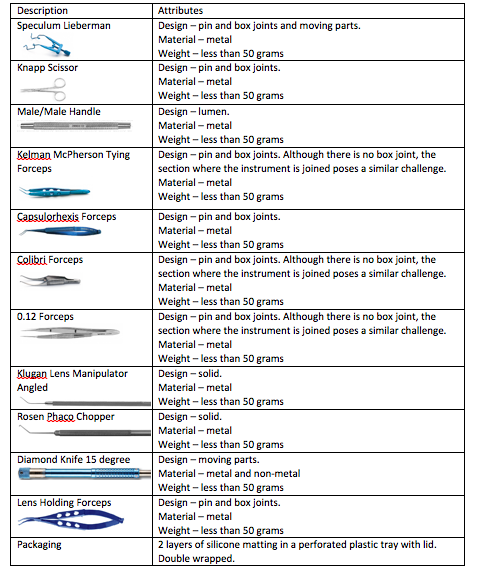
![Quick Guide to assembling a dental tray [AS4187 product families]](https://blog.scancare.com.au/hubfs/Tables,_Charts_forms/dental_tray_assembly_table.png)
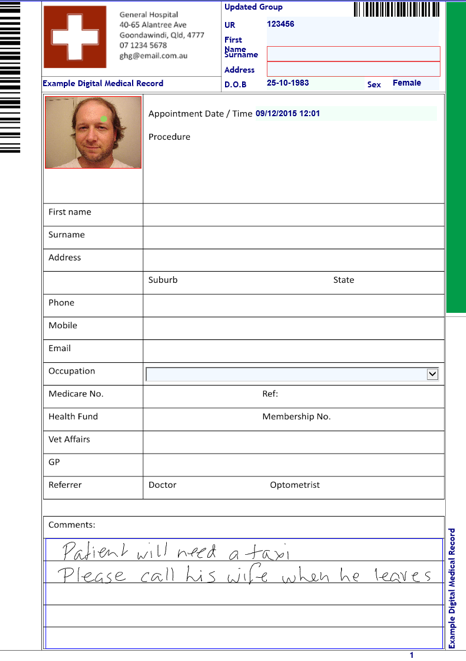

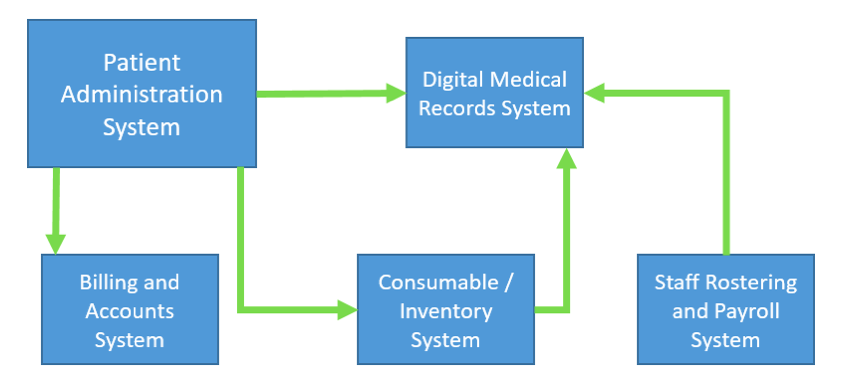













![How to transition to paperless medical records systems. [3 Tips]](https://blog.scancare.com.au/hubfs/lady_photocopying.jpg)