Infection control in body art and piercing clinics
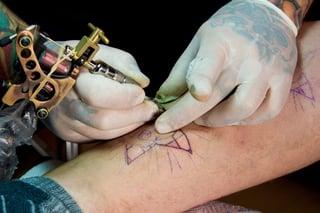 Body art and personal appearances services are a fast growing industry and we are experiencing increased downloads of our eBook on surgical instrument tracking by owners and managers of body piercing clinics and body art studios. So I want to bring these types of businesses and services into the discussion on infection control and sterilizing standards. Particularly the use of re-usable devices used for skin penetration.
Body art and personal appearances services are a fast growing industry and we are experiencing increased downloads of our eBook on surgical instrument tracking by owners and managers of body piercing clinics and body art studios. So I want to bring these types of businesses and services into the discussion on infection control and sterilizing standards. Particularly the use of re-usable devices used for skin penetration.
Each state and territory in Australia has in place some form of guideline or legislation covering the infection control requirements for body art and piercing clinics. All of them make reference to either AS 4815 or AS 4187, but most of them mention AS4815:2006 which is the standard that applies to office-based health care facilities, specifically the reprocessing of reusable medical and surgical instruments and equipment as well as the maintenance of the associated environment.
Given that most of these type of businesses are based in office based facilities it is logical that AS4815:2006 is referenced. It’s also worth noting that in Queensland and Tasmania piercing practitioners must by law have completed the course ‘HLTIN402C- Maintain Infection Control Standards in Office Practice Settings’
So what then does this mean for your office based personal appearance service?
What are the compliance requirements for body art and piercing businesses?
If you own or manage a body art or piercing business then, you must ensure your operation is compliant with a number of legislation, guidelines and standards.
Make compliance with your state legislation your foremost priority. Then, consider how you can comply with the relevant standards and, finally, the guidelines. So firstly, ascertain what your state's legislation is around the provision of these services. Then, apart from the specific requirements in Queensland and Tasmania I referred to above, the minimum requirement is mostly likely compliance with AS 4815 2006.
What is AS 4815?
AS4815 is an Australian and New Zealand standard that covers the re-processing of reusable surgical instruments. The standard was written for and applies to office based health care facilities but the scope of the standard covers skin penetration establishments and is therefore relevant for a body piercing clinic. It covers how to clean, disinfect, sterilize and store your re-usable surgical devices and how to maintain the re-processing environment.
My aim with this blog is to give you a basic overview of the requirements of the standard (AS4815), so that you have an understanding of what is required.
Cleaning and handling of used items
‘All used items shall be cleaned in a designated area to prevent possible contamination of processed items’.
What this means is that there needs to be a degree of physical separation between clean and dirty areas.
Packaging and wrapping of items prior to sterilization
‘The routine sterilization of unwrapped critical* instruments for immediate use is unacceptable.’
*The Spaulding classification defines critical instruments as those that enter or penetrate into sterile tissue, cavity or bloodstream.
Any instrument used to penetrate skin must be sterilized in a wrapped state. The purpose of packaging and wrapping items is to provide an effective barrier against contamination.
Quality Management
Quality management is about establishing policies and procedures as well as keeping records and product identification as well as traceability. You need to keep records for a minimum of 7 years.
‘Procedures should be in place to link sterilizer cycle batch information of a critical item that has been sterilized, to the patient.’
You must keep a record of which instruments were used on your clients and be able to track them back to the date they were sterilized, the sterilizer that was used as well as the Sterilizer cycle.
‘For each sterilizing cycle, records shall be maintained. The sterilizing cycle record shall include—
(a) the date of cycle;
(b) the sterilizer number or code (if there is more than one sterilizer within the office-based practice);
(c) the cycle or load number (if more than one load per day);
(d) the exposure time and temperature;
(e) the name or identification of the person authorizing release of load contents;
(f) the specific contents of the load, e.g. instrument trays, bowl sets; and
(g) the ‘read out’ or result of physical, chemical or biological/enzymatic indicators that are used.’
Storage
‘Storage areas for sterile items shall be controlled to prevent contamination and shall be dedicated for that purpose only.’
This means you can’t just leave instruments on a desk or table. There needs to be a dedicated area for the purpose of storing processed items. Shelving should be positioned at least 250mm above floor level and at least 440mm below ceiling fixtures.
Conclusion
Compliance can be quite daunting for a small office based body art and personal appearance service. I hope that with this blog I have given you a better idea of what may be involved.
If you want to find out more, your primary source of information and advice should be the Federation of Sterilizing Research and Advisory Councils of Australia (FRASCA).
A copy of AS/NZS 4815 2006 can be purchased from SAI global.