How to categorize a Cataract Tray for Ophthalmic Centres/Ophthamology
Surgical Instrument Product Families for Ophthalmologists

The new version of AS/NZS 4187 released on the 15th December last year brought some very significant changes to the previous standard. One of the most significant changes is the standards reference to Product Families.
In a previous blog, I provided an overview of ISO/TS 17665-3:2013 - Sterilization of health care products - Moist heat - Part 3: Guidance on the designation of a medical device to a product family and processing category for steam sterilization.
Today I will get into the specifics of how to apply the standard to a Cataract Tray. Let's take a look at how to do that.
How to prepare a Cataract Surgery Tray
The diagram below shows the makeup of a typical Cataract Tray:
Using our Guide to Product Family Categorisation, we will set the attributes for each instrument.
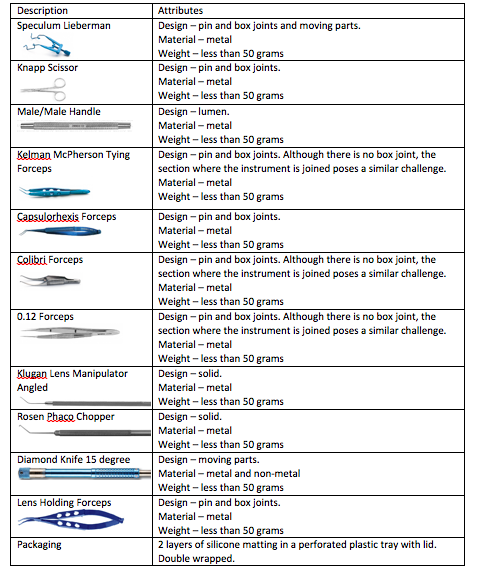
From the table above we can deduce an overall description: small low weight metal and non-metal instruments with pins and box joints, and moving parts supported on 2 layers of silicone matting in a perforated plastic tray with lid. Double wrapped.
This is how it looks in our free downloadable matrix:
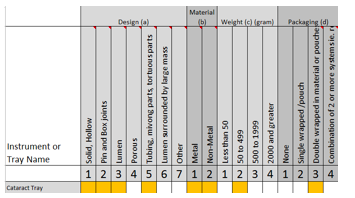
Given that we need to select the attributes that pose the greatest challenge to sterilization, the outcome would look like this:
Design (a5)
Material (b2)
Weight (c2)
Packaging (d3)
From here you will require a copy of ISO/TS 17665-3:2013 - Sterilization of health care products - Moist heat - Part 3: Guidance on the designation of a medical device to a product family and processing category for steam sterilization. It can be ordered from SAI Global.
Table 6 in the standard provides a matrix which allows you to identify the Product Family and Steam Penetration Resistance. Unfortunately I can’t reproduce the table due to copyright, but I can however show you the results.
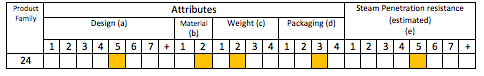
So our Cataract Tray has a Steam Penetration Resistance of (e5) and belongs to Product Family #24.
Surgical Instrument Product Families Simplified
Once you get the hang of it, the categorisation process is a reasonably straightforward process. The matrix and the examples here as well as in the standard itself, will help you make solve the puzzle.
One thing to remember is that the categorisation process is quite subjective and therefore the way person A categorises instruments and trays can be differently to person B.
AS/NZS 4187 does not provide guidance on product families. However it does state that the method used to classify an RMD into a product family shall be based on similar attributes to those discussed above and should be documented.
It also states that reference should be made to the manufacturer’s instructions.
This will be the first in a series of blogs demonstrating how to apply the standard to specific types of Trays. Next in the series – a Dental Extraction Tray