How to make sense of the AS4187 categorisation of product families
 The new version of AS4187 (AS/NZS 4187) released on the 15th December last year brought some very significant changes to the previous standard. One of the most significant changes is the standards reference to Product Families.
The new version of AS4187 (AS/NZS 4187) released on the 15th December last year brought some very significant changes to the previous standard. One of the most significant changes is the standards reference to Product Families.
The standard states that the method used to classify an RMD into a product family shall be documented.
RMD’s can be grouped into Product Families based on:
- materials of construction
- its intended use
- design characteristics
- its physical mass and thermal conductivity.
The idea being that an RMD or set of RMD’s (Tray) is exposed to a re-processing regime that ensures that it can be safely and effectively reprocessed.
However, the standard offers no guidance on how to classify product families except that it makes reference to ISO/TS 17665-3:2013 - Sterilization of health care products - Moist heat - Part 3: Guidance on the designation of a medical device to a product family and processing category for steam sterilization.
While ISO/TS 17665-3 Part 3 provides excellent guidance on how to do this, it is still a complex document so the purpose of this blog is to provide an overview of the standard and hopefully shed some light on the classification process, so that when you pick up the standard you will find a degree of familiarity with the terminology and process.
Classification
Classification is done based on the four General Attributes of an RMD and each attribute is given a unique code.

Design
The design of an RMD varies significantly. For example: "Is it hollow?" or "Does it have pins and box joints or does it have moving parts?". The standard breaks up RMD design into seven design elements and assigns a code to each.
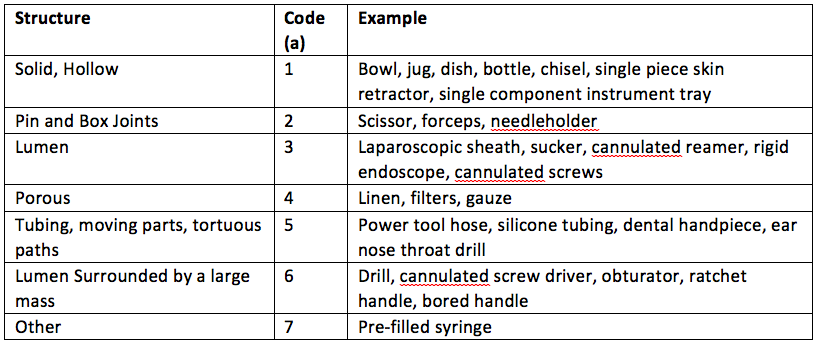
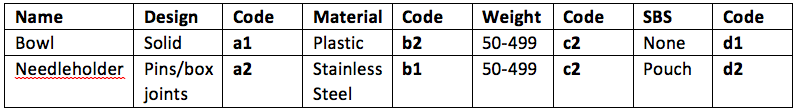
If for example, an RMD was a bowl or a dish it would have a design attribute of ‘a1’, whereas if the RMD was a needleholder it would have a design attribute of ‘a2’
Material
Material is easy because there are only two material elements.
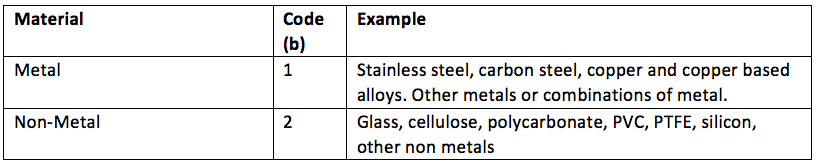
If for example, an RMD was a bowl, it may be plastic and therefore have a material attribute of ‘b2’, whereas a needleholder is stainless steel and would have a material attribute of ‘b1’
Weight
Weight is also fairly straight forward as determining the weight on an RMD is a technical exercise.
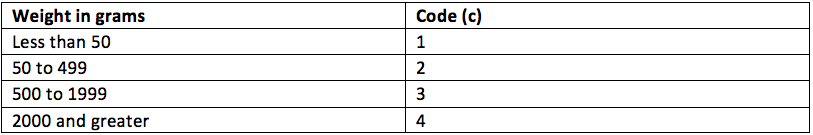
In this case both the bowl and the needleholder may be between 50 to 499 grams so both would have a weight attribute of ‘C2’
Sterile barrier system / packaging system
There are four sterile barrier system elements
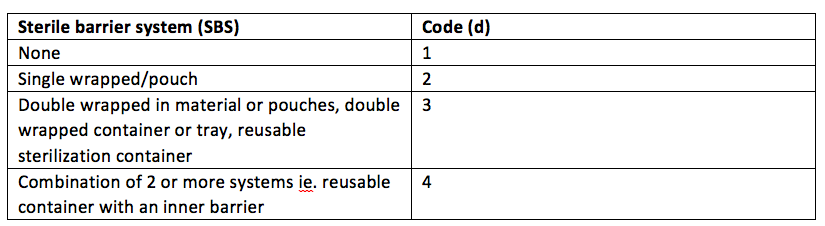
So in a small day surgery, a bowl may be sterilised un-wrapped in a bench top sterilizer and would have an SBS attribute of ‘d1’
If our needleholder on the other hand was wrapped in a single pouch, it would have an SBS attribute of ‘d2’
So this is what we have so far:
Steam Penetration Resistance
The steam penetration resistance is subjectively estimated from the attributes identified for each RMD. The higher the attribute code number the higher the steam penetration resistance. For RMD’s grouped together in trays, the steam resistance is estimated based on the RMD / SBS that creates the greatest challenge to sterilization. For sterilizing loads containing multiple RMD’s and sets, the steam penetration resistance is estimated on the RMD / SBS or set that creates the greatest challenge.
There are 8 levels of Steam penetration resistance from 1 to 7, anything higher is grouped as 7+
Once the Steam penetration resistance is estimated, the RMD is given a unique Product Family Number.
Conclusion
Because steam penetration resistance is a subjective estimation, the way one sterilizing department categorises RMD’s may be quite different to another.
While AS/NZS 4187 may not provide guidance on product families it does state that the method used to classify an RMD into a product family shall be based on similar attributes to those discussed above and should be documented.
It also states that reference should be made to the manufacturer’s instructions.



