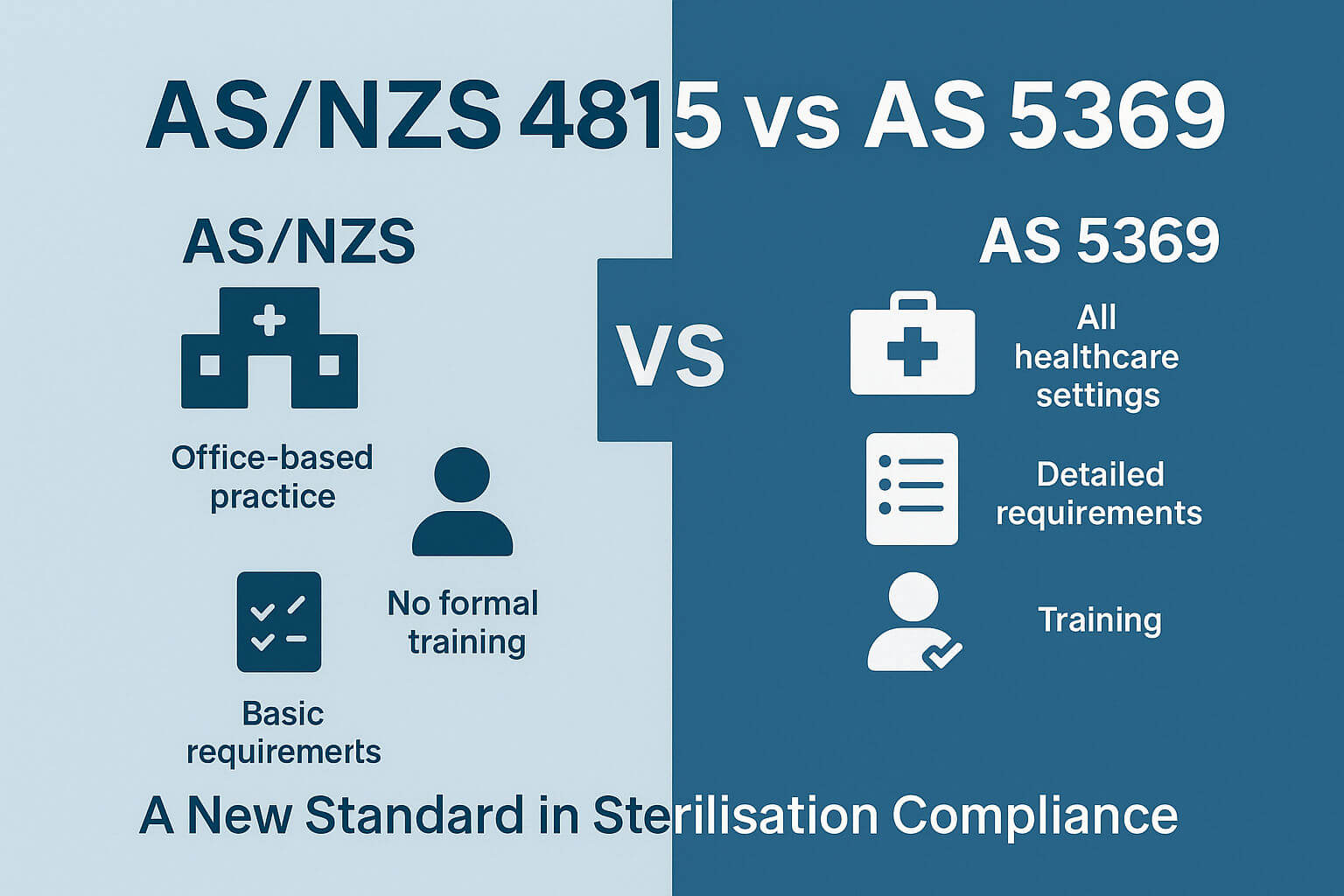
Top 10 Key Differences Between AS/NZS 4815 and AS 5369
Introduction
If your clinic or healthcare facility is still using AS/NZS 4815:2006, you're working under an outdated framework. The newly introduced AS 5369:2023 is a modernised, risk-based standard designed to enhance infection control and sterilisation practices in Australia. Below, we compare the top 10 differences between AS/NZS 4815 and AS 5369, with a focus on documentation compliance, staff training, and reprocessing protocols.
Read on to discover the Top 10 differences between the standards.


![Quick Guide to assembling a dental tray [AS4187 product families]](https://blog.scancare.com.au/hubfs/Tables,_Charts_forms/dental_tray_assembly_table.png)