Simplifying AS4187 Product Families
 Blog posts and downloads related to product families blogs are by far the most accessed content on our website. By a long way.
Blog posts and downloads related to product families blogs are by far the most accessed content on our website. By a long way.
And it’s with good reason. The concept of product families can be extremely complicated and confusing.
In this blog, I’ll take another look at ‘ISO/TS 17665-3:2013 - Sterilization of health care products - Moist heat - Part 3: Guidance on the designation of a medical device to a product family and processing category for steam sterilization’ and work our way through the complexity.
Classifying an Instrument Set
The ISO standard says that the product family assigned to a collection of instruments (Set/Tray) should be determined by the medical device which presents the greatest challenge to the sterilization process (Steam Penetration Resistance - SPR).It goes on to say, ‘The Product family assigned to a procedure set should normally align with the medical device or component assigned the highest steam penetration resistance unless influenced by adjacent medical devices and / or components.’
So, for the large part, the steam penetration resistance value should correspond with the Product Family number.
Therefore, if an instrument set has a Steam Penetration Resistance of one (1), its product family number should also be one (1).
Given that there are only seven (7) SPR values given in the standard, it would seem that there would only be seven (7) Product families.
Obviously, this is not the case as the standard defines 29 Product Families.
The ISO standard provides a table (Table 6) that identifies these 29 different product families.
The standard recognises however, that the table is not exhaustive and that further product families will need to be created by comparing the instrument set or instrument to a similar one that already has a product family value.
In fact, there are a number gaps in the table that don’t provide PF values at all.
For example, a single metal instrument with box joints weighing less than 50 grams and wrapped in a single pouch is not defined in the table. Amazing, given how common these are. Scissor, Needleholder etc.
So clearly, classifying instrument sets into product families in not a straight forward technical exercise.
i.e A + B = C
And this is where it becomes complicated and confusing. The key point is that the SPR value should normally align with the product family value, unless influenced by adjacent medical devices or components.
This little word, ‘unless’ turns the classifying process from a straight forward technical exercise into a subjective exercise.
That’s OK, but it means you must have an incredibly intimate knowledge of the ISO standard and to make judgement calls on many instruments and instrument set configurations.
This obviously amounts to a massive amount of work to classify your instruments and instrument sets.
Why is it so complicated?
Section 5.2 of AS4187:2014 provides some guidance on the requirements for implementation of Product Families.
The requirements are similar to those in ISO standard:
- Description (metal, non-metal)
- Intended use
- Design (lumens, moving parts)
- Physical characteristics (mass, thermal conductivity
- Packaging
AS4187 notes that ‘ISO/TS 17665-3 and ISO 17664 provide useful information to assist in assigning an RMD to a product family.’
The important point here is that you do not need to use the ISO standard to assign items to product families. You can devise and document your own method based on consideration of the above requirements in AS4187.
I come from a technical background (mechanical and software engineering), so when resolving problems or designing products, my thought process always comes back to following a technical (scientific) process.
A + B = C
So why not apply a purely technical process to the method of assigning product families?
Let’s wind back to a previous quote from the ISO standard:
‘The product family assigned to a procedure set should normally align with the medical device or component assigned the highest steam penetration resistance unless influenced by adjacent medical devices and / or components.’
For me, the key to coming up with a system, is the first part of the statement:
‘The product family assigned to a procedure set should normally align with the medical device or component assigned the highest steam penetration resistance’
Put simply, if an instrument set has a Steam Penetration Resistance of one (1), its product family number should also be one (1).
And that’s it!
Do you need 29 product families? Well maybe you do, but let’s start with seven and go from there.
Product Family Matrix
We have devised a product family matrix based on the principles of the ISO standard and the assumption that the SPR is determined by the medical device or packaging method that presents the greatest challenge to sterilization.
It takes the highest value attribute from design, material, weight and packaging and uses an algorithm to determine the SPR and the product family.
In the image below, you can see in this Cataract Tray, that the design attribute of ‘Tubing and moving parts’ presents the greatest challenge to sterilization.
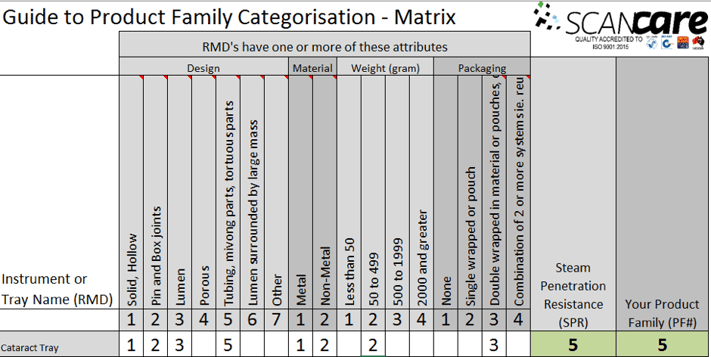
Therefore, this Cataract Tray has an SPR of 5 and Product family of 5.
You can download our product family matrix for free. It includes a six-part video series that demonstrates its use with the provided examples of a cataract tray, a dental assessment set, a general ortho set, a single needleholder and a single diathermy hook.
Conclusion
The Process of assigning Instruments and instruments sets to Product Families doesn’t need to be complicated. In my view, the simpler the better.
I am sure many of you have already devised your own methodology and it would be great if you could share that in the comments section below this blog.
If not, please feel free to download our Product Family matrix and watch the videos.



