How to prepare for AS4187 product families compliance deadline
 With the deadline for implementation of AS4187 2014 coming up fast, I thought that it may be worthwhile to take another look at product families.
With the deadline for implementation of AS4187 2014 coming up fast, I thought that it may be worthwhile to take another look at product families.
There seems to be a lot of interest in AS4187 and product families and my blog of August 2015 is by far our most read page on our website. The helpful categorisation guide gets downloaded several times each week.
As a quick background; the standard was published on 15 December 2014 and the recommendation is that the standard be implemented within 2 years of publication. So with the deadline looming I thought it may be useful to take a look at the parts of the standard that relate to product families and how they may help you prepare.
Definition of Instrument Product Families
‘Groups or subgroups of product characterized by similar attributes such as mass, material, construction, shapes, lumens, SBS or packaging system and which present a similar challenge to the cleaning, disinfecting and/or sterilizing processes.’
The interesting thing about this definition is its reference to groups or subgroups of product.
If you process a Reusable Medical Device (RMD) by itself, then it will have a product family deduced from the attributes of that RMD and its Sterile Barrier System (SBS) alone. However that changes when you process RMD’s as a group (tray). In that case it is the attributes of the RMD that provide the greatest challenge to the sterilizing processes which determines the product family.
But when you process a group of trays and/or singles together, it becomes the tray or single that provides the greatest challenge to sterilization that determines the type of cycle used.
Policies and Procedures
With regard to product families, the standard states that, at a minimum, procedures are required for:
‘Validation and requalification of cleaning, disinfecting and sterilizing processes including the rationale used to assign a particular RMD to a specific product family and processing category.’
The standard states that when assigning an RMD to a Product Family and to a method of reprocessing that the following shall be considered and documented:
- A description of the RMD, including the materials of construction (e.g. metal, non-metal combinations, plastics) and its configuration.
- The intended use of the RMD.
- The design of the RMD, including design characteristics that can affect selection of a cleaning, disinfecting or sterilizing process, e.g. ease of disassembly and assembly, tolerance to moisture, heat and chemicals, presence of lumens, moving parts, fibreoptics, electronics.
- The physical characteristics of the RMD, including its mass, surface area and thermal conductivity.
- Packaging of the RMD, including the SBS for sterilized RMDs.
Given that you need to document the above information perhaps you could use that information to devise the rationale you are going to use to assign RMD’s to product families.
If you were to tabulate the above requirements and use your answers to determine the challenge to sterilization, then perhaps you could design your own rationale based on that. For example:
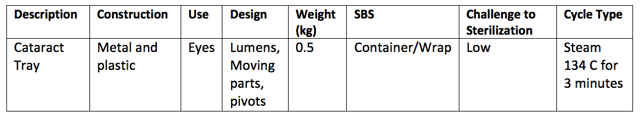
It’s worth noting also that the requirements contained in AS4187 are very similar to those used in ISO/TS 17665-3 to form the basis of the rationale it uses.
Our guide to product family categorisation, may be of assistance in documenting these requirements. It provides a table containing the required columns and allows you to select each attribute and determine the product family.
It’s based on ISO/TS 17665-3 Guidance on the designation of a medical device to a product family and processing category for steam sterilization which is the ultimate resource for information on assigning an RMD to a product family.
It’s worth noting here that ISO/TS 17665-3 standard relates to steam sterilization only, however the definition (above) of product families provided in AS4187 doesn’t appear to restrict the categorization of RMD’s to steam sterilization processes only.
ISO/TS 17665-3, however could still provide useful guidance for other sterilization/disinfection procedures.
It’s important also to note that the standard says that reference should be made to the manufacturer’s instructions for use. So it would be worthwhile seeking advice from the manufacturer regarding the appropriate Product family and re-processing conditions for their RMD’s.
Conclusion
The concept of devising a rationale to determine an RMD’s product family may appear quite daunting.
I certainly don’t profess to be an expert on the subject by any means, but by using a structured methodology it can become somewhat more of a technical exercise.
Obviously when it comes to determining the challenge to sterilization and the cycle type, you’ll need to use your knowledge and experience.
And of course, it would make sense to seek advice from the manufacturer whenever you can and utilise whatever help they can provide.



