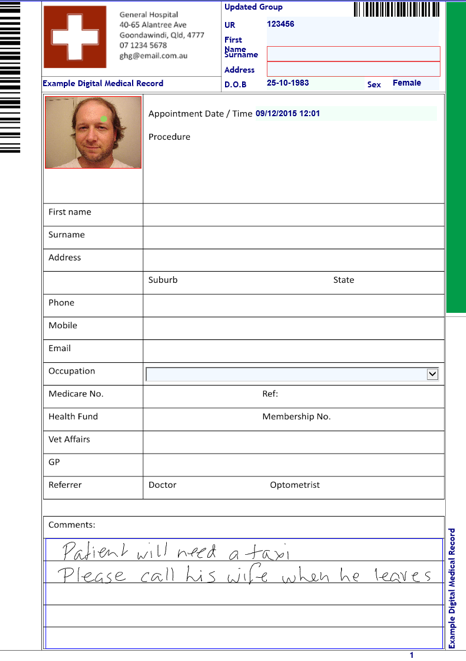
What are the minimum label requirements under AS4187?

Re-usable Medical Devices must, in accordance with AS4187 2014, be labelled prior to sterilisation. The label must identify the contents and provide information for batch control.
But while the standard's requirements are critical, there are also a number of other requirements such as OH&S that may affect the way you label your RMD’s.

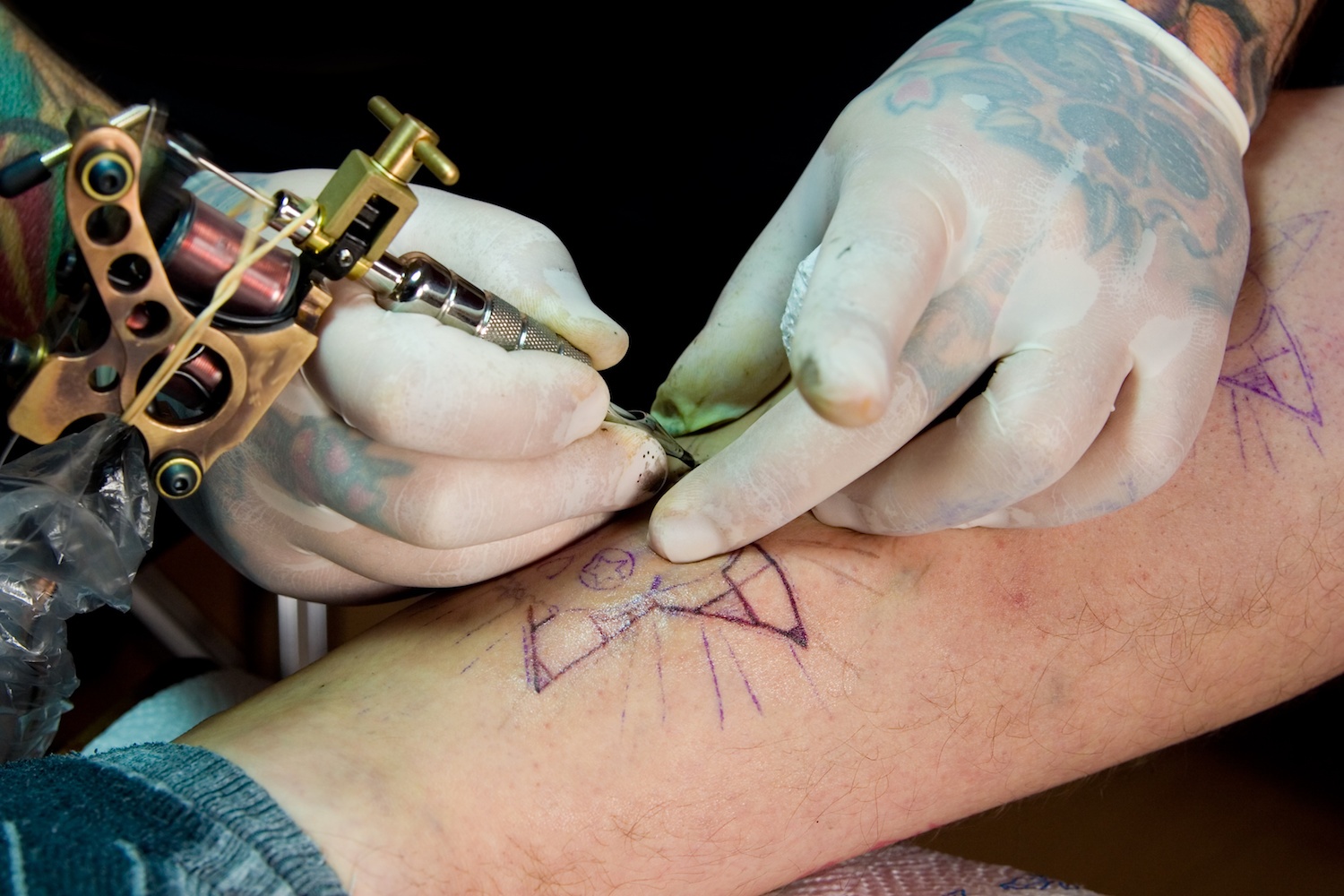

 Complying with Australian standards AS4187 or AS4815 can be a difficult task for any health care facility, but for office based health care facilities, physical space limitations can make it even more difficult. Finding space to wash, wrap and sterilize surgical instruments can be tricky for the smaller facilities and bench space is typically limited so finding space to put things can be a challenge.
Complying with Australian standards AS4187 or AS4815 can be a difficult task for any health care facility, but for office based health care facilities, physical space limitations can make it even more difficult. Finding space to wash, wrap and sterilize surgical instruments can be tricky for the smaller facilities and bench space is typically limited so finding space to put things can be a challenge.

![Quick Guide to assembling a dental tray [AS4187 product families]](https://blog.scancare.com.au/hubfs/Tables,_Charts_forms/dental_tray_assembly_table.png)