Video: What AS4187 means for office based healthcare

In August 2015 we wrote about the implications of the new AS/NZS4187 standard on office based healthcare facilities. It is the second best read article on our blog and we have now created snapshot video that covers the main points. See below.


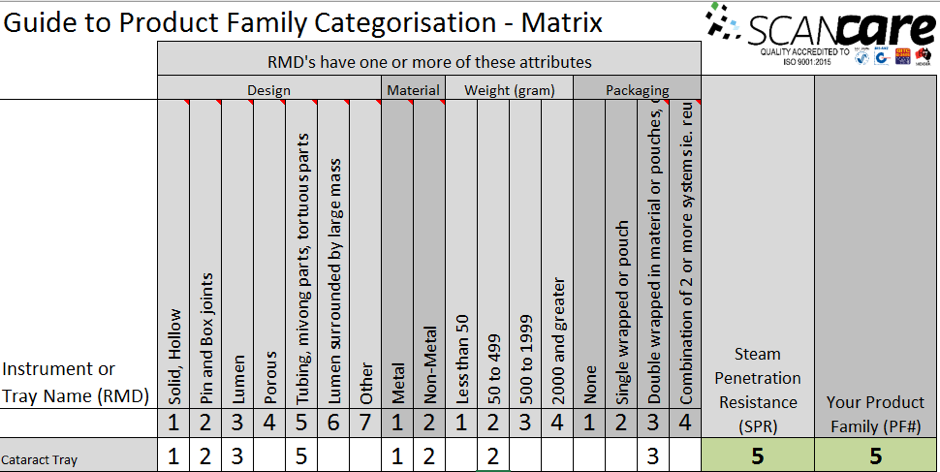

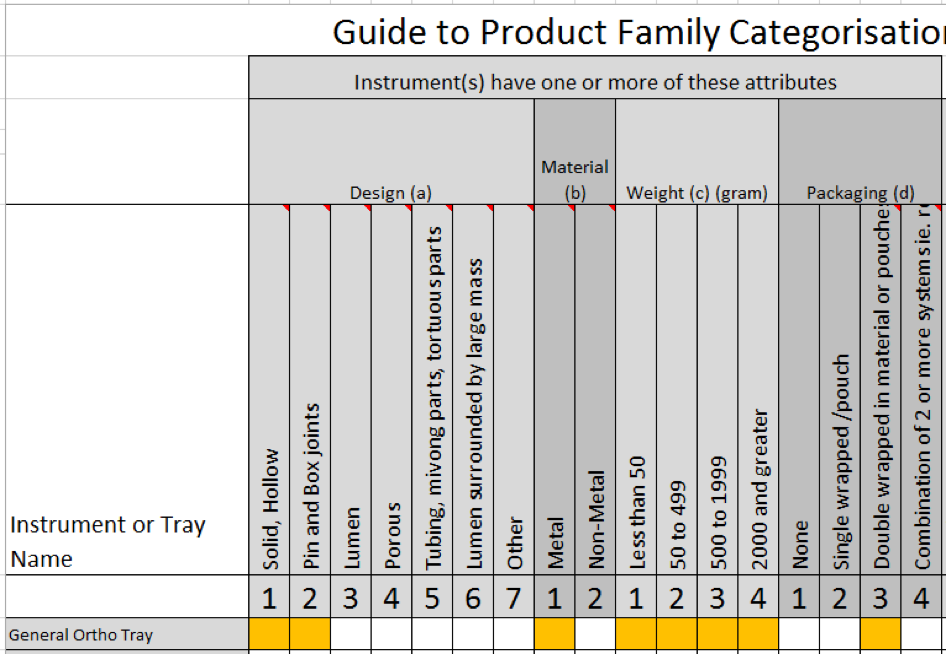



 Complying with Australian standards AS4187 or AS4815 can be a difficult task for any health care facility, but for office based health care facilities, physical space limitations can make it even more difficult. Finding space to wash, wrap and sterilize surgical instruments can be tricky for the smaller facilities and bench space is typically limited so finding space to put things can be a challenge.
Complying with Australian standards AS4187 or AS4815 can be a difficult task for any health care facility, but for office based health care facilities, physical space limitations can make it even more difficult. Finding space to wash, wrap and sterilize surgical instruments can be tricky for the smaller facilities and bench space is typically limited so finding space to put things can be a challenge.![Quick Guide to assembling a dental tray [AS4187 product families]](https://blog.scancare.com.au/hubfs/Tables,_Charts_forms/dental_tray_assembly_table.png)