How to categorize a Cataract Tray for Ophthalmic Centres/Ophthamology
Surgical Instrument Product Families for Ophthalmologists
The new version of AS/NZS 4187 released on the 15th December last year brought some very significant changes to the previous standard. One of the most significant changes is the standards reference to Product Families.
In a previous blog, I provided an overview of ISO/TS 17665-3:2013 - Sterilization of health care products - Moist heat - Part 3: Guidance on the designation of a medical device to a product family and processing category for steam sterilization.
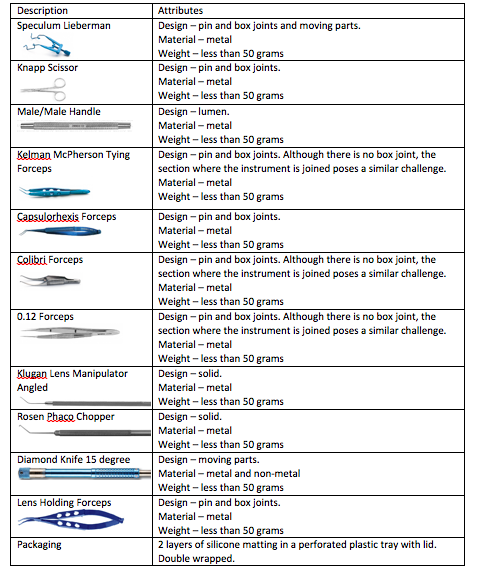
Today I will get into the specifics of how to apply the standard to a Cataract Tray. Let's take a look at how to do that.