CSSD Revolution: the Impact of complex instruments on resources
The advances in surgical procedures and surgical instrumentation, over the past 10-20 years has been astounding. Once upon a time, just about every surgical procedure was open surgery and involved simple pivot based stainless steel instruments. These days, surgical instruments are highly complex hybrid material devices.
This has led to a quiet revolution in sterilizing departments around the world. Instruments were once simple and tray assembly straightforward.


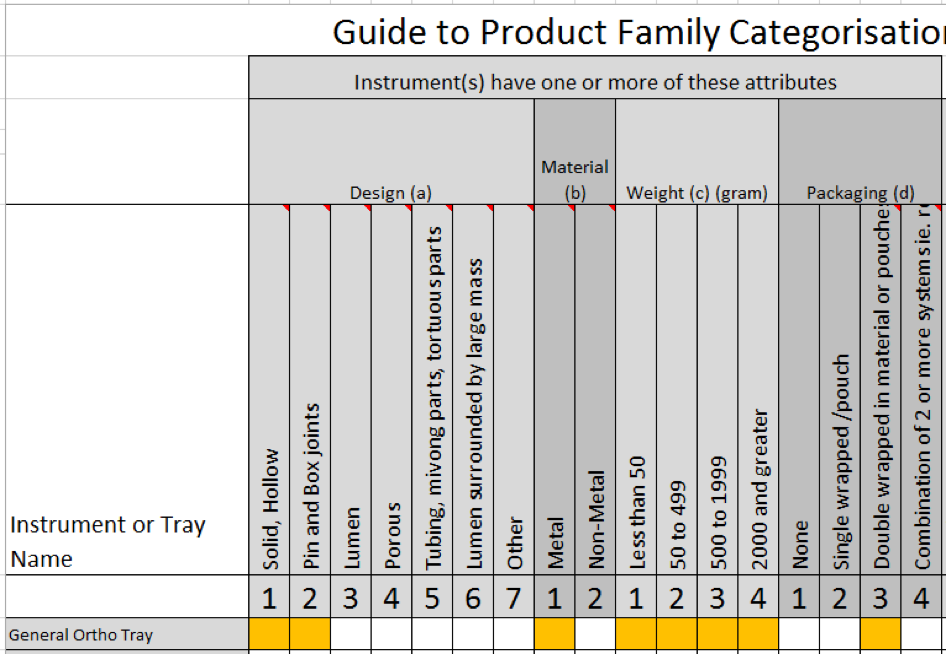



 Complying with Australian standards AS4187 or AS4815 can be a difficult task for any health care facility, but for office based health care facilities, physical space limitations can make it even more difficult. Finding space to wash, wrap and sterilize surgical instruments can be tricky for the smaller facilities and bench space is typically limited so finding space to put things can be a challenge.
Complying with Australian standards AS4187 or AS4815 can be a difficult task for any health care facility, but for office based health care facilities, physical space limitations can make it even more difficult. Finding space to wash, wrap and sterilize surgical instruments can be tricky for the smaller facilities and bench space is typically limited so finding space to put things can be a challenge.![Quick Guide to assembling a dental tray [AS4187 product families]](https://blog.scancare.com.au/hubfs/Tables,_Charts_forms/dental_tray_assembly_table.png)