AS4187 Product Families: How to categorize a General Orthopaedic Set
The new version of AS/NZS 4187 released on the 15th December last year brought some very significant changes to the previous standard. One of the most significant ones being the standards reference to Product Families.
In my blog Here's how to make sense of the AS4187 categorisation of instrument product families, I provided an overview of ISO/TS 17665-3:2013 - Sterilization of health care products - moist heat - Part 3: Guidance on the designation of a medical device to a product family and processing category for steam sterilization.
This blog is the third in series where I'm going into the specifics of how to apply the standard to a number of different instruments sets.
This time we take a look at a general orthopaedic set. The diagram below shows the makeup of a typical a General Orthopaedic Set:
Using our downloadable ‘Guide to Product Family Categorisation’ we will set the attributes for each instrument.
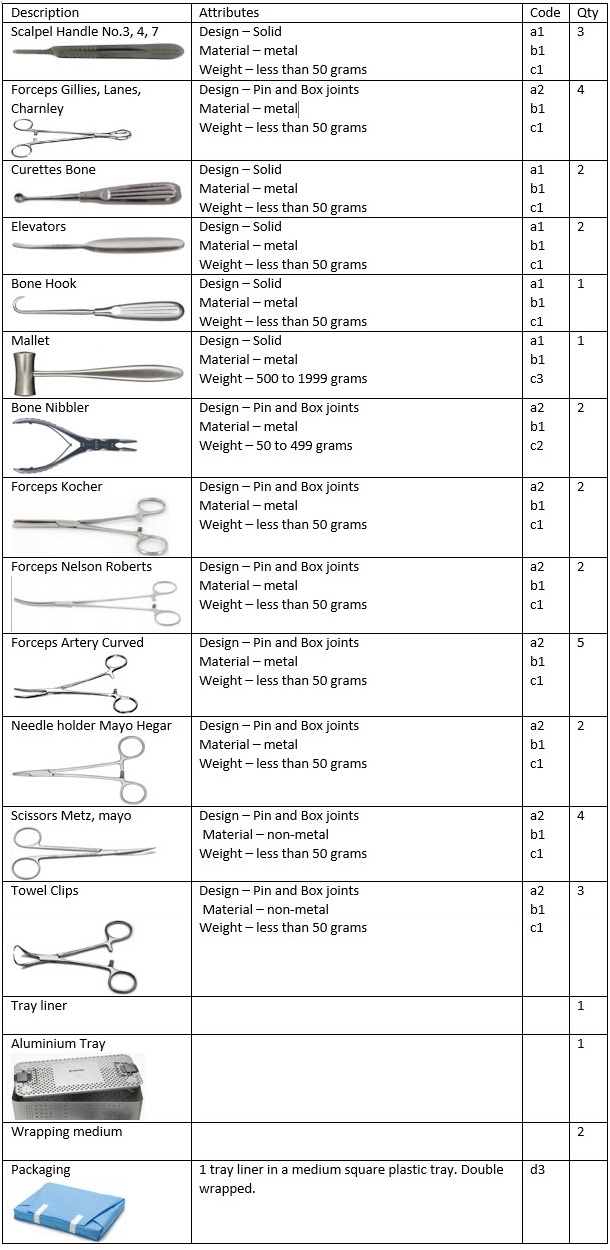
From the table above we can deduce an overall description: high weight metal instruments of solid, and pin and box joint construction supported on a Tray liner in a large perforated aluminium tray. Double wrapped. Materials used comprise metal only. The tray is made from aluminium. Instruments with pin and box joints pose the greatest challenge due to the close matting surfaces.
This is how it looks in our free downloadable matrix:
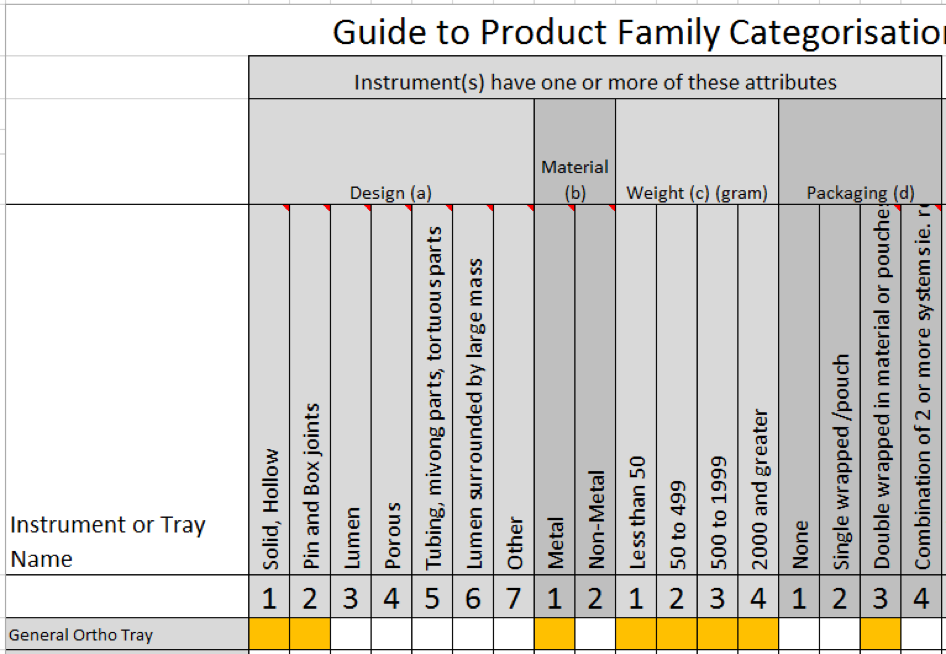
Given that we need to select the attributes that pose the greatest challenge to sterilization, the outcome would look like this:
- Design (a2)
- Material (b1)
- Weight (c4)
- Packaging (d3)
Note that the combined weight of all items is 2000 and greater so that’s why I have selected c4 as the weight attribute.
OK, so this is where you are going to need a copy of ISO/TS 17665-3:2013 - Sterilization of health care products - Moist heat - Part 3: Guidance on the designation of a medical device to a product family and processing category for steam sterilization.
Table 6 in the standard provides a matrix which allows you to identify the product family and steam penetration resistance. Unfortunately I can’t reproduce the table due to copyright, but I can however show you the results.
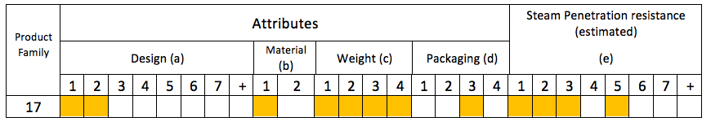
The Steam penetration resistance (SPR) is estimated like this:
- Solid items have a SPR of e1
- Pins and box have a SPR of e2
- The SBS has a SPR of e3
However, due to the high weight of the tray the SPR has been increased to e5
So our tray has a steam penetration resistance (SPR) of (e5) and belongs to product family #17.
Conclusion
Once you get the hang of it, the categorisation process is a reasonably straightforward process and with the help of the matrix and the examples here and in the standard, you should be able to work it out.
Just remember that the categorisation process is quite subjective and one person may categorise instruments and trays differently to another.
Sometimes there will be no match at all and you will need to define your own product family.
While AS/NZS 4187 may not provide guidance on product families it does state that the method used to classify an RMD into a product family shall be based on similar attributes to those discussed above and should be documented.
It also states that reference should be made to the manufacturer’s instructions.


![Quick Guide to assembling a dental tray [AS4187 product families]](https://blog.scancare.com.au/hubfs/Tables,_Charts_forms/dental_tray_assembly_table.png)