AS 5369:2023 Product Families Online Course
Introduction
We are proud to announce the Scancare Academy and our very first course, Understanding & Implementing Product Families, is available now and its completely free to enroll.
Background
AS 5369:2023 requires that RMD's be assigned to Product Families. The purpose is to group RMD's together that present a similar challenge to cleaning, disinfecting and sterilizing processes.
In this course we use concepts and methods from ISO/TS 17665 Part 3 - Guidance on the designation of a medical devices to a product family and processing category for steam sterilization as the basis for how we assign RMD's to Product Families.
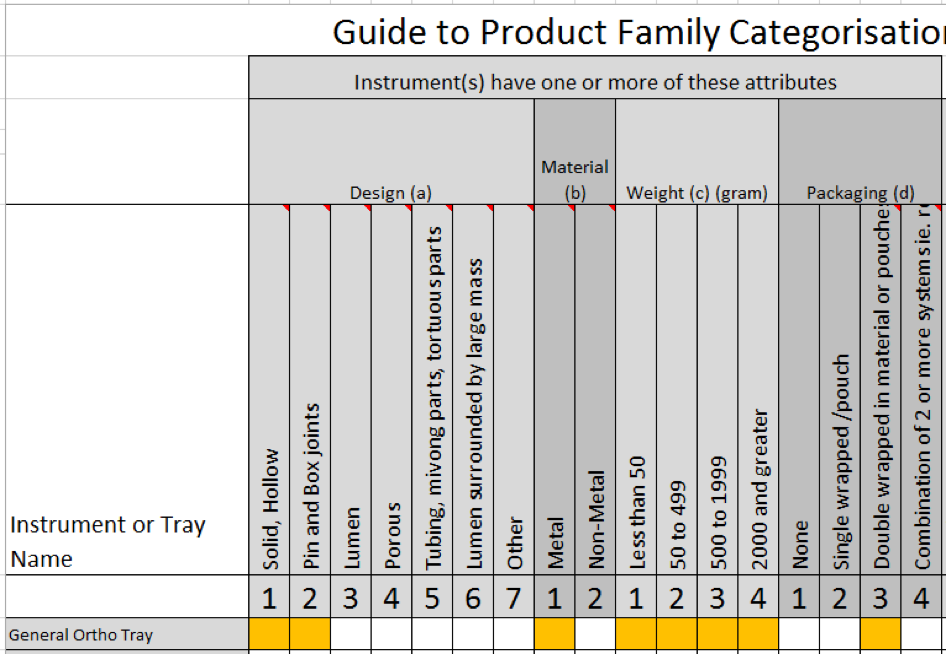
The ISO specification uses certain features of instruments such as the instruments weight, the material it is made of, how its designed and the Sterile Barrier system used to assign it to a Product Family.
We take a look at these different instrument features and see how they are used to determine product families.
We will also be using our free Product Family Matrix to work through a number of examples of Instruments and Trays.
Course Curriculum
- Welcome to the course
- Chapter 1 - General Instrument Attributes
- Chapter 2 - Detailed Attributes
- Chapter 3 - Steam Penetration Resistance
- Chapter 4 - Assigning Instruments to Product Families
- Chapter 5 - The Matrix
- Chapter 6 - Master Product
The course only takes 2-3 hours to complete.
By completing this course you will gain an understanding of the requirements of ISO/TS 17665-3 and AS 5369:2023 as they relate to Product Families.
What you learn will give you the tools and knowledge needed to get you on your way you to implementing Product Families in your facility.
So click the button below and check it out.