AS 4187 Product Family categorisation - it's only part of the story

In my last blog, we looked at the complex and confusing nature of Product Families and looked at a way to simplify the categorisation process for assigning instruments and instrument sets to product families.
In it we provided a free Product Families Toolkit containing a matrix and a six-part video series, demonstrating how to use it.
If you read my previous blog and got the free product families toolkit, then you have you have things underway, but that’s only part of the story.
Ultimately, the purpose of categorising instruments and instrument sets into product families is to ensure that they are safely and effectively processed. This means:
- That the integrity of instrument(s) is not compromised
- That the instruments(s) are effectively processed
This presupposes the concept of ‘compatible’ processing cycles. An instrument should not undergo an incompatible process.
For example, AS4187 lists common holding times for thermal disinfection and saturated steam.
Some Reusable Medical Devices (RMD) may be damaged, or not achieve effective processing if processed through a particular cycle type.
However, some may be compatible with several different cycles types.
Therefore, assigning product families to compatible cycle types and processing methods is a logical step.
But even more important than that, is preventing RMD’s from being processed in incompatible processing types.
It’s one thing to have a list of compatible processing types. You can do that in an Excel spreadsheet, but it is entirely another thing to ensure that only compatible items go through compatible processes.
So how do you do that?
Product Labelling
AS4187 has requirements for labelling prior to sterilization.
‘Labelling shall identify the contents and provide information for batch control.’
Therefore, you are using a method to label your product. If you're using a manual tracking system, that may mean simply writing the name of the contents on the sterilizing tape, or if you are using an electronic tracking system, that will most likely be a label with a barcode on it. In my blog What are the minimum label requirements under AS4187? , I wrote about the requirements of the standard, and commented on what else may be useful to have on your product labels.
To prevent RMD’s from going through incompatible processing types, you will need to know which RMD’s are compatible with what cycle types. Possibly the best way to do that is to have that information on your product label.
In the example below, you can see the Product Family (PF), the Steam Penetration Resistance (SPR) and the Cycle Type (TYPE) are displayed.
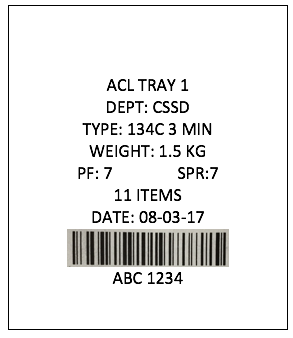
So that’s a great first step, but it still doesn’t stop you physically putting it through an incompatible processing cycle.
In my blog AS4187:2014 – Do paper based tracking systems still cut it? I spoke about how I had met a number of delegates at the WFHSS conference in Brisbane last year who felt that it was becoming increasingly difficult to meet the requirements of AS4187:2014 with their existing manual tracking systems. The amount of manual record keeping required was becoming extremely burdensome.
Preventing RMD’s from going through incompatible processing types will be another challenge for those of you using manual systems.
Those of you who have an electronic tracking system may be at a significant advantage if your system can detect and alert you to RMD’s allocated to incompatible processes.
Conclusion
The idea that RMD’s can be processed using several different ‘Compatible’ processing cycles means that you need to be able to correlate a product family with one or more compatible reprocessing methods.
In closing, it’s important to note that the classification of RMD’s to product families applies to all methods of re-processing. Not just steam sterilizing. It also applies to cleaning, disinfecting, and other forms of sterilization such as low temperature sterilizing. It also applies to endoscope reprocessing.
Assigning RMD’s to Product Families is one thing but physically preventing an RMD from being reprocessed in an incompatible process is another thing all together.


