Has Spaulding Classification had its day?

I recently attended the 37th Annual Dental Congress in Melbourne and it was fantastic to catch up with the dental community and talk about the infection control issues that affect them. We had many visitors to our booth and had several enriching discussions with progressive thinking delegates who were passionate about infection control.
One discussion in particular caught my interest, as the delegate had very interesting views regarding the Spaulding Classification and how it was applied in dentistry. Our discussion centred around whether the classification and AS4815 were still relevant and whether AS4187 should be the "go to" standard for office based dental practices instead.
What's the background?
Earle Spaulding wrote a paper in 1939 that proposed a strategy for defining the sterilizing and disinfection requirements for surgical devices.
He proposed that the level of sterilization/disinfection should be determined by how the device was to be used.
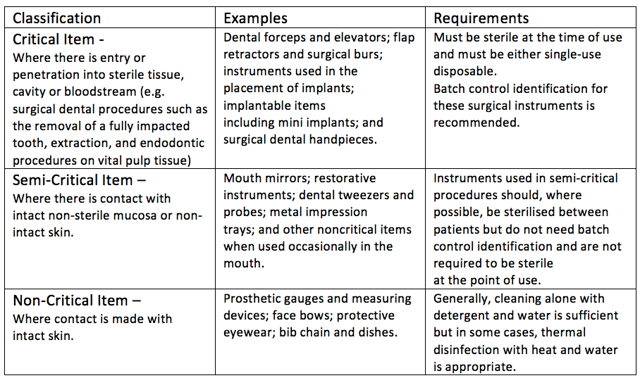
He came up with three classifications as per the table below:

This classification system has been widely adopted and used as a reference in most infection control related standards and guidelines.
ASNZS 4187:2014 defines the Spaulding Classification in Section 5.1.2 – Classification for processing.
Interestingly however, the ASNZS 4815:2006 standard doesn’t refer to the Spaulding Classification at all, but does however adopt its principles.
Application to Dental
The Australian Dental Association Guidelines for Infection control third Edition 2015 specifies the application of the Spaulding classification to dental equipment and instruments in Section D – Instrument reprocessing.
What’s very useful here is that the document provides relevant examples and requirements for equipment and items for each of the three classifications.
But back to that discussion
OK, so what’s wrong with that? It’s all pretty straightforward really. Right?
Well it is for the most part, but what if...?
Remember that semi-critical items:
- should be sterilised where possible
- don’t need to batch tracked
- aren’t required to be sterile at the point use.
So, what about where a dental mirror, tweezers and a probe are being used in a procedure where there is penetration into sterile tissue, bleeding occurs and the mirror, tweezers and probe get blood on them?
Should the semi-critical items now be treated as critical items? It’s a valid argument given that they were used in a procedure using critical items and covered in blood.
So, say for example, you decide, that the blood covered items do need to be treated as critical items and be washed and sterilised. You probably have several instruments of the same type, so once the procedure is done and the items returned for reprocessing and washed to remove gross soil, how do you identify which ones should be treated as critical or semi-critical?
They all look the same, right? And how do you track that?
Now, wind the conversation forward and what do you do if a patient presents at your clinic hepatitis positive and believes that they contracted the infection from a critical procedure performed by you.
How do you prove that the instruments used on them went through a validated sterilizing process?
But hang on, you only have batch tracking records for the critical stuff. Remember the semi-critical items with blood on them? You don’t have batch tracking records for them because it’s not required and they don’t have to be sterile at the point of use.
How do know that the virus wasn’t transferred to those?
Conclusion
Perhaps that open and frank conversation I had that day at conference should be had by everyone interested in Infection Control.
The main points of the conversation:
- Is the Spaulding classification still relevant, appropriate, safe?
- AS4815 is now 11 years old, the world has changed and become much more litigious. Is it still relevant, appropriate, safe?
- Is AS4815 too lenient regarding its requirements for limited level batch tracking.
- Why don’t you just sterilize and batch track everything? Wouldn’t that be easier, safer?
- Why do we need two standards covering reprocessing of surgical devices?
Electronic batch tracking is not expensive, in fact it’s probably cheaper than what it currently costs you if you're using batch labelling sticker guns and books or manually typing sterilizing results into the patient record. It's certainly more efficient, will save you time, give you traceability at the click of a button and provide comfort knowing that you are tracking to the highest level.